
What are Generic Drugs?
Generic Drugs are those drugs which are marketed under a non-proprietary or approved name rather than a proprietary (Patent) or brand name. Generic drugs are equally effective and inexpensive compared to their branded counterparts. E.g.:- Crocin contain the molecule Paracetamol and market with the brand name of crocin while generic version also contain molecule Paracetamol and market with the name of paracetamol only.
What is meant by Patent?
It’s a government authority or license conferring a right or title for a set period, especially the sole right to exclude others from making, using, or selling an invention.
Why Generic drugs are cheaper?
Generic makers don’t face the same costs as manufacturers of brand-name drugs as they often invent the drug, a process that can cost hundreds of millions of dollars. The rationale behind drug patents: They give pharmaceutical companies a period of years when only they can make money on a product in which they have made a large investment. That investment also includes advertising. For a generic manufacturer, no such investment is required — not in development and not in marketing. The drug’s formula is known, the clinical trials are complete; the generic maker’s only requirement is to demonstrate to regulators that its version is as good and effective in humans as the original. That is an enormous economic advantage for these companies, which is why their drugs are much cheaper.
Are Generic Drugs as effective as branded drugs?
Yes, The generic drugs have similar efficacy and therapeutic value as that of the branded medicines.
How do generic medicines benefit the patients?
By taking generic medicines, a patient can save up to 90% on your medical expenses. As the medicines are available at an affordable price.
Why do brand-name drugs look different from their generic versions?
Government Patent Law do not allow a generic drug or medicine to look exactly like other drugs already on the market. Generic medicines and brand-name medicines share the same active ingredient, but other characteristics, such as colors and flavourings, that do not affect the performance, safety, or effectiveness of the generic medicine, may be different.
What standards must generic medicines meet to receive FDA approval?
FDA's Office of Generic Drugs reviews the application and make sure the generic medicine with the following standard; • The active ingredient in the Generic drug is the same as that of the brand-name drug/innovator drug • The generic medicine is the same strength • The medicine is the same type of product (such as a tablet or an injectable) • The medicine has the same route of administration (such as oral or topical • It has the same use indications • Generic drug companies must submit evidence that all the ingredients used in their products are acceptable, and FDA must review that evidence • (Except Color, flavouring etc. which does not affect the performance of the medicine) • It lasts for at least the same amount of time • It is manufactured under the same strict standards as the brand-name medicine.
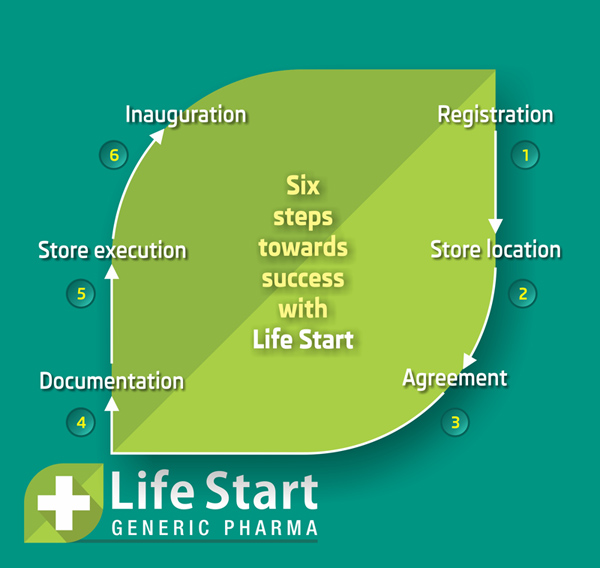
"Feel free to ask us if you have more questions in your mind."

